Abstract
Introduction: The 2014 revised International Myeloma Working Group (IMWG) definition of multiple myeloma (MM) and smoldering MM (SMM), reclassified high risk patients with SMM who had one or more of bone marrow plasma cell percentage (BMPC) ≥ 60%, involved to uninvolved free light chain ratio (FLCr)≥100 and >1 focal lesion on magnetic resonance imaging as MM. A previous model developed at our institution to predict progression of SMM to MM or systemic amyloidosis included serum-M spike ≥3 g/dL, BMPC≥10% and FLCr >8 as predictors and was devised before 2014 (Dispenzieri et al. Blood; 2008). We aimed to devise a simple scoring system, using routinely used parameters, to risk stratify patients with SMM as defined by the current diagnostic criteria.
Methods: We retrospectively reviewed electronic medical records to identify patients who satisfied the 2014 revised IMWG criteria for SMM, who were seen at our institution between 2001 and 2015. We collected data regarding baseline characteristics, date of progression, myeloma defining event, and first treatment. Time to progression (TTP) was defined as time duration from diagnosis of SMM to initiation of therapy for MM/amyloidosis. Patients were censored in TTP analysis if they did not progress at last follow-up, started cancer chemotherapy or steroids for other indications, or if they enrolled in a treatment trial of SMM. We used receiver operating characteristics (ROC) analysis to identify best cut-offs for continuous variables like serum M-spike, BMPC and FLCr in predicting progression at 3 years from diagnosis of SMM. We used Cox proportional hazards model to identify the effect of predictors on TTP and estimate their hazard ratios (HRs). TTP was estimated using Kaplan Meier method.
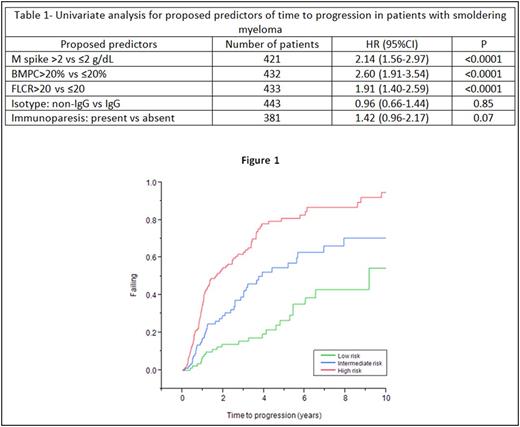
Results: We included 433 patients in the final analysis. The estimated median follow-up duration was 6.0 years (95%CI, 5.4-6.8). Using ROC curve analysis, we identified M-spike of 2g/dL, BMPC of 20%, and FLCr of 20 as cut-offs to predict progression at 3 years. Apart from the above variables, we also included immunoglobulin isotype (IgG vs non-IgG) and presence of immunoparesis in the univariate analysis. The results of univariate analysis are shown in Table 1 . M-spike >2 g/dL, BMPC>20%, and FLCr>20 were significant in the univariate model and were included in the multivariate Cox proportional hazards model which included 419 patients. M-spike [HR: 1.67 (CI, 1.18-2.38); p=0.003], BMPC [HR: 2.02 (CI, 1.44-2.84); p<0.0001] and FLCr [HR: 1.88 (CI, 1.37-2.57); p=0.0001] predicted reduced TTP in the multivariate model. Depending on the presence of M-spike>2 g/dL, BMPC>20% and FLCr>20, we divided patients into three groups- low risk (none of the three risk factors; n=143), intermediate risk (one of the three risk factors; n=121) and high risk (two or more of the risk factors; n=155). The estimated median TTP for the low, intermediate and high risk groups were 9.1 years (CI, 5.4-not reached), 3.7 years (CI, 2.8-6.9) and 1.6 years (CI, 1.1-2.5) respectively (p<0.0001; Figure 1). There was no difference in TTP between patients who had two or three of the above risk factors at diagnosis. The 5-year estimated risk of progression in the low, intermediate and high risk groups were 26.5%, 57.1% and 81.0% respectively.
Conclusions: M-spike >2 g/dL, BMPC>20% and FLCr>20 at diagnosis are routinely measured parameters which can be used to risk stratify patients meeting current diagnostic criteria for SMM. Presence of 2 or more of these risk factors identifies a high risk group with shorter TTP. These patients might benefit from clinical trials of early intervention to prevent/ delay progression to MM/amyloidosis.
Dispenzieri: Celgene, Millenium, Pfizer, Janssen: Research Funding. Gertz: Millennium: Consultancy, Honoraria; Celgene, Novartis, Smith-Kline, Prothena, Ionis, Amgen: Honoraria. Dingli: Karyopharm Therapeutics: Research Funding; Millenium: Consultancy; Takeda: Consultancy; Janssen: Consultancy; Alexion Pharmaceuticals: Consultancy. Russell: Imanis Life Sciences: Equity Ownership; Vyriad: Equity Ownership. Kapoor: Takeda, Celgene and Amgen: Research Funding. Kumar: Celgene, Millennium, BMS, Onyx, Janssen, Noxxon, AbbVie, Amgen, Merck, Oncopeptides, Skyline Diagnostics, Takeda: Consultancy; Celgene, Millennium/Takeda, Onyx, AbbVie, Janssen, Sanofi, Novartis, Amgen, Genentech, Merck, Oncopeptides, Roche, Skyline Diagnostics: Research Funding; Skyline: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal